Grade 6
Physical Sciences
Chapter 9: Classifying Matter
Lesson 1: Physical Properties Lesson 2: Elements and Compounds Lesson 3: Solids, Liquids and Gases Lesson 4: Water and MixturesLesson 1: Physical Properties
Matter is anything that has mass and volume (occupies space).
The amount of matter in an object is called Mass. Mass can be measured in milligrams (mg), grams (g) or kilograms (kg). An object's mass remains the same.
Weight is a measure of the pull of gravity on an object. Therefore the weight of an object changes depending on the gravity. You would weigh less on the moon than you would weigh on Earth because the moon has less gravitational pull.
Volume: Volume describes the number of cubes that can fit inside an object. It is a measure of how much space an object occupies. Therefore the object needs to have a 3 dimensional structure. Here, we introduce the concept of height (H). If you are measuring the volume of an ice cube that measures 2cm on all sides, the volume is; V = L X W X H = 2cm X 2cm X 2cm = 8 cm 3. Realise that the formula to calculate the volume of an object can be shortened to V = A (of the base) X H or short V = Base X height. This is important because the same formula applies if the object is based on rectangular sides or if the base is of a different shape. A 3D shape with a circular base is called a cylinder.
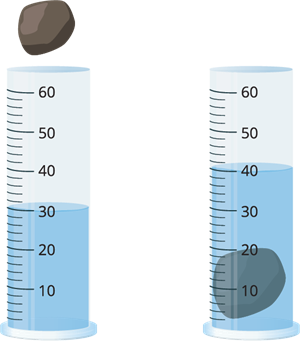
To find the volume of an irregularly shaped object, you can use water in a measuring container. First add the water, measure how much water is in the container. Then surmerge the object until it has all been covered by the water then record the new reading of the volume of water. The volume of the object is the difference between the new final reading and the original reading. This is called displacement. A solid object will displace an amount of water that is the same as its own volume.
States of Matter
Matter can be found in three common states namely: solids, liquids and gases.
Solids: Solids have a shape and take up a definite amount of space. In solids, the particles of matter are packed tightly and mostly in a regular pattern. The pencil, pen, book, desk, blocks, wood, ice ... are all solids.
Liquids: Liquids do not have a definite shape, they take the shape of the container. Juice is a liquid, if you pour it into a glass, it will spread out and take up the shape of the glass. In liquids, the particles that make up matter are farther apart and can move more freely than in solids. Water, juice, milk, and oil are examples of liquids.
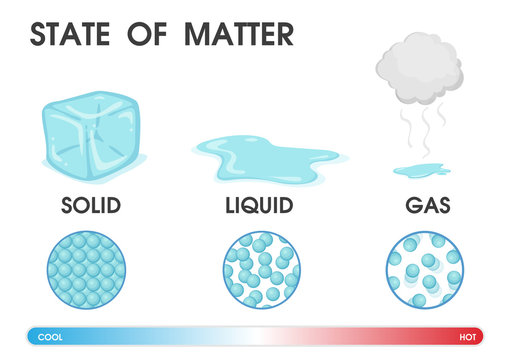
Gases: If you pour juice into a glass, it goes to the bottom of the glass makes the glass half full. Gases do not have a definite shape. In addition, if you put a gas into a container, it spreads out throughout the container. In gases, the particles spread out so as to fill the space in the container. If the space is small, the particles will be tight together, if the space is big, the particles will be spread out farther apart. Air is mostly made out of gases.
Density: Density is the amount of matter per unit space (volume). Remember we said solids have particles that are tightly packed and gases have particles that are far apart, this corresponds with how much matter is in a unit volume, so solids are expected to have more matter per unit volume than gases. This also means solids are expected to have a higher density than liquids, and liquids have a higher density than gases. in addition to this, objects of the same state can have different densities. As in, different solids can have different densities. The amount of matter is measured using Mass and is expressed in grams (g). To calculate the density of an object, divide its mass by its volume, M ÷ V. If the mass is in grams and the volume in cubic centimeters, then the result will have units in grams per cubic centimeter (g/cm3).
Bouyancy: When you put an object in wter, you feel it being pushed upwards. For example, if you try to push a baloon or abeach ball inside a swimming pool, you will feel the water pushing the ball out. The upward force ofa liquid or gas is called bouyancy. It is the force tht enables objects to float on water and is used to design boats and ships.
Archimedes’ principle states that buoyant force is equal to the weight of the fluid that is displaced. The size of the fluid’s buoyant force determines whether an object sinks or floats. If the buoyant force exceeds the object’s weight, the object floats. For example, buoyant force pushes an ice cube back toward the surface of the water in a glass. Since the buoyant force is greater than the weight of the ice cube, the ice cube floats. Archimedes’ principle explains why ships can float on water and balloons can float in the air.
Physical Properties
The physical properties of a substance are properties that can be observed without changing the identity of the substance. These properties help us tell substances apart. Density, color, hardness, odor, magnetism, boiling point, and texture are some physical properties.
Insulators and Conductors: Insulators are materials that do not transfer thermal energy well. As in, they prevent the transfer of thermal energy. One good use of insulators is in making handles for cooking pans/pots. Wood is a good example of an insulator. In humans and animals, fat acts as an insulator. Fat prevents the transfer of thermal energy, which is why bears gain fat just before winter so as to prepare them to concerve more thermal energy during winter.
Conductors (kinda as the name suggests) are materials that can effectively conduct heat, as in they are good at transferring thermal energy. Most metals are good conductors of heat, which is why cooking pots are made from metals. Clay is a poor conductor of heat, which means it takes longer to get hot but also takes longer to lose the heat (cool down), this property makes clay materials good for slow cooking.
Lesson 2: Elements and Compounds
What is Matter made of?
Everything around you is made of elements, or pure substances that cannot be broken down into any simpler substances. Elements can be solids, liquids, or gases.
The 'lead' in a pencil is actually composed of the element carbon. Water is composed of the elements hydrogen and oxygen. Have you heard people call water H2O? H is the symbol for hydrogen, and O is the symbol for oxygen. The number 2 indicates that there are two hydrogen particles for each oxygen particle in water. Every element has a symbol that is one or two letters long. Hydrogen (H) and oxygen (O) have one-letter symbols. Some elements have two-letter symbols. For example, the symbol for copper is Cu.
Most elements combine to form compounds. A compound is a substance formed from the chemical combination of two or more elements and the compound acts as a unique substance. For example, hydrogen and oxygen are gases but their compounds such as H2O is a liquid.
Many years ago, people thought that all matter was made up of either Earth, Water, Air and Fire. We now know that matter is made up of elements. An element is a substance that is made up of only type of matter. Because an element is made of one type of matter, you cannot break it down into any simpler form. Hydrogen, Oxygen, Gold, Silver are some examples of elements. There are several other examples of elements.
Atoms: An atom is the smallest part of an element. You can think of atoms as particles. However, atoms are very tiny and you cannot see them with your naked eye. All atoms in an element are alike, for example, all atoms in silver are alike, and you can describe them as Silver atoms. Same for hydrogen and gold and other elements. The atoms of a certain elemet are different from the atoms of another element.
Atoms are too small to see without a high-powered microscope and because it took time for microscopes to become powerful, the structure of the atom was not correctly described until the late 1900s.
Atoms have a nucleus, whic is at the center of an atom and contains most of its mass. The nucleus contains neutrons and protons. Both have approximately the same mass. Neutrons have no electrical charge; they are neutral. Protons have a positive electrical charge.
An element is identified by the number of protons contained in the nucleus of each of its atoms. Every element has a unique number of protons and is defined as the element's atomic number.
The atomic mass of an element is defined and calculated as the sum of the number of protons and neutrons. Atoms of the same element may have different numbers of neutrons, which means they will have different atomic mass. Atoms of the same element that have different atomic masses are called isotopes. If you look at a detailed periodic table, you will notice that an isotope’s atomic mass is listed beside its name or symbol.
Atoms also contain electrons. Electrons are negatively charged and have a very low mass. The number of electrons in an atom does not change its atomic number or atomic mass because of their small mass. The negative charge of the electrons is attracted to a proton's positive charge.
If there are more electrons than protons, an atom has an overall negative charge. If there are more protons than electrons, an atom has a positive charge. A charged atom is called an Ion.
Periodic Table: Because of the diverse differences between elements, scientists found it necessary to develop a way to organize the elements, so that elements with similar properties can be grouped together. About 150 years ago, a scientist named Mendelev developed a Periodic Table a s way to organize elements. See the periodic table below:
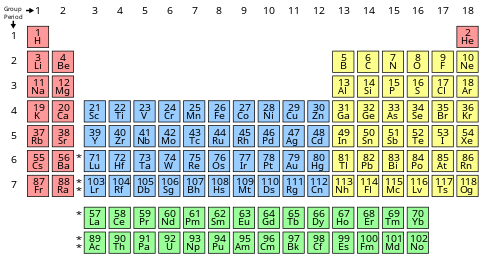
Columns and Rows: As shown on the periodic table, elements are organized into rows and columns. Elements in the same column are said to be in the same Group. Elements in the same row are said to be in the same Period. When Mendeleev arranged the elements in a table, he saw a pattern. Elements with similar properties were grouped near one another. Scientists use these patterns to predict how an element behaves. Hydrogen (H), for instance, reacts easily with other substances. The elements that share its column in the periodic table also react easily. Elements that share the same row often have similar properties, too. Iron (Fe) is magnetic. Find Iron in the Periodic table. Notice that the two elements next to iron are also magnetic.
Metals and Nonmetals: A metal is shiny, it can be bent or hammered into shape. examples of metals include Iron, Aluminum and Copper. Metals allow heat and electricity pass through them (i.e., they conduct heat and electricity). Thats why Aluminum and Iron are used for cooking pots and Copper is used for Electric wires.
Metalloids are elements that have some of the properties of metals but not all.
Nonmetals is a large group of elements euch as Nitrogen and Hydrogen (and many others). They have none of the properties of metals.
Compounds
A Compound is formed when two or more elements combine chemically. Iron is a gray metal. Oxygen is a colorless / clear gas. Water is also clear/colorless. When Iron reacts with Oxygen, in the presence of moisture, to form Rust, it changes color to a brown solid.
Here are a few examples of compounds.
- Water (liquid) - combination of Hydrogen (gas) and Oxygen (gas)
- Table salt (solid) - combination of Sodium (Na) (solid) and Chlorine (Cl) (gas).
- Rust (solid) - combination of Iron (Fe) (solid) and Oxygen (O) (gas) in the presence of water/moisture.
- Sugar, starch, glucose are all compounds of Carbon, Hydrogen and Oxygen (C H O) with each element in different proportions to make the different compounds.
A chemical formula is a simple way to indicate the composition of elements in a compound. It shows the number and types of atoms in a compound. Formulas help scientists categorize and label chemicals. For example, the chemical formula for sodium chloride is written as NaCl. This means that it is a compound made from sodium (Na) and chlorine (Cl). This formula also indicates that for every sodium atom there is one chlorine atom. The two elements exist in a 1:1 ratio.
A molecule is the smallest particle of a compound that still has all the qualities of that compound.
Lesson 3: Solids, Liquids and Gases
Most matter can be a solid, liquid or gas depending on the temperature. Temperature is a measure of how hot or cold something is. It is a measure of the average amount of kinetic energy of the atoms and molecules in a material. When you hang clothes to dry, the heat energy from the sun heats up the water in the clothes causing it to evaporate (change from liquid to gas (vapor). A change from one state to another involved either loss or gain of energy by the substance. When matter gains energy, its particles move faster resulting in more collisions between particles. The body uses up heat energy from the surface of the skin to evaporate sweat, this loss of heat energy is intended to make you feel cooler. Sweating, therefore, is a biological process which allows you to cool down when its hot outside.
The state of matter can change in other ways. Sublimation occurs when a substance changes directly from a solid to a gas without going through a liquid state. Ice cubes left in your freezer for a long period of time will become smaller. Eventually, the ice cubes will 'disappear'. This is because the water molecules in the ice cubes change directly from the solid state (ice) to the gaseous state (water vapor).
If you heat a solid substance, the particles move faster and if there is enough energy, the solid will melt into a liquid. Melting is the change of state from solid to liquid. If you heat the liquid farther, the particles move even farther apart and the liquid changes to a gas. This process is called Boiling. The temperature at which a liquid becomes a gas is its boiling point. Evaporation is that process where a liquid can change to a gas without boiling. Water on ponds, lakes, rivers and oceans is constantly undergoing evaporation.
Cooling occurs when we take away heat energy from a substance. This makes the particles move closer together. If we cool a gas into a liquid, we call that Condensation. Or we can say the gas condenses to a liquid. If we cool a liquid into a solid (like if we put water into the freezer), we call that Freezing, or we can say the liquid freezes into a solid. Water freezes into ice.
Temperature and Volume
If you put a balloon into very cold water, it shrinks. The number of air molecules inside the balloon has not changed. Temperature has caused the volume of the gas inside the balloon to change. Because the balloon is now much colder, the molecules move more slowly. The spaces between the molecules decrease, and the air inside the balloon has a smaller volume.
Other Physical Changes
Dissolving is the process that occurs when the molecules of a solid move apart and are separated by the molecules of a liquid. Heat usually speeds up this process. Heat causes the different molecules to move around faster, so they mix more rapidly.
Lesson 4: Water and Mixtures
A mixture (as you probably already guessed) is a combination of two or more kinds of matter. However, in a mixture, each kind of matter keeps its properties. For example, a salad is a mixture of various vegetables and dressing. But each vegetable remains in its original state.

When two substances are mixed together and they blend completely so you can no longer detect the individual substances, we call this a Solution.
A mixture of two metals (or a mixture that contains at least one metal) is called an Alloy. Some alloys are stronger than the elements used to make it. Bronze is an alloy or Copper and Tin, and it is stronger than either of them. Steel is an alloy or Iron and carbon.
Other Types of Mixtures
A suspension is a mixture made of parts that separate upon standing. To make a suspension, add fine sand to a bottle of water. Shake it, and watch the particles move. Soon, the sand particles will separate from the water and settle to the bottom of the bottle. You can separate a suspensio by filtration.
An emulsion is a suspension of two liquids that usually do not mix together. Emulsions are stable homogeneous mixtures of very small droplets suspended, rather than dissolved, in a liquid.
A colloid is a stable homogeneous mixture in which very small, fine particles of one material are scattered throughout another material, blocking the passage of light without settling out. Fog is a liquid-in-gas colloid. Smoke is a solid-in-gas colloid. Nonfat milk is a solid-in-liquid colloid.
Solutions
A solution is a mixture of one substance dissolved in another. The properties of a solution are the same throughout the mixture. Solutions are similar to colloids. Both are homogeneous mixtures, but the particles in solutions are smaller than those in colloids.
Solutions have two parts. The solute is the substance that dissolves. The solvent is what the solute dissolves in.
Solubility is a physical property of solutes in different solvents. It describes the amount of a substance that can dissolve in a specific solvent.
Many metals form substances called alloys. Alloys are mixtures of one or more metals with other solids. Steel is an alloy made mostly of iron and carbon. Bronze is an alloy of copper and tin, brass is an alloy of copper and zinc.
Mixtures can be separated using various methods. For example, you can allow the substances to settle. Settling is when the matter with a higher density moves down to the bottom of the container and the matter with the lower density stays at the top. If you add sand into water, the sand will settle at the bottom.

Filtration is a method to separate mixtures based on size. You can filter water using a sieve, the larger particles stay in the sieve and the smaller particles pass through the sieve and are collected as filtrate.
Magnetic attraction: Magnets are often used to separate scrap metal in a junk yard. A magnet will attract elements such as iron, nickel and cobalt.

One method to separate substances in a solution is to use distillation. Distillation relies on boiling points. In distillation, a solution is heated until the liquid that has a lower boiling point becomes a gas. The solid or the liquid that has a higher boiling point is left behind. The gas is passed slowly through a tube where it slowly cools down (condenses) and goes back to a liquid state and settles in another tube. This is the method used to separate gasoline fro curde oil.
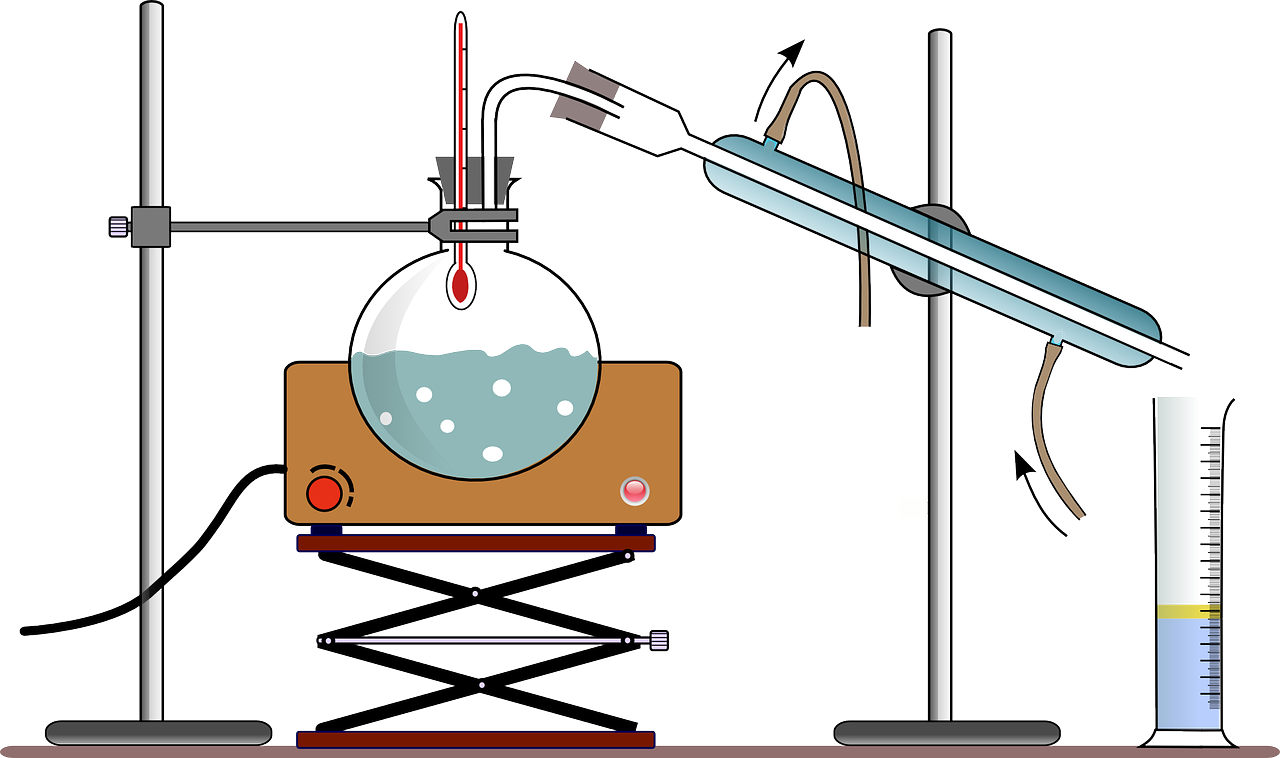
Evaporation can also be used to separate a solution into its its parts. This is the process used to make salt from sea water. When you allow sea water to evaporate, you are left with solid salt. This technique differs from distillation in that; 1. it doesnt involve boiling, 2, we are aiming to collect the solid, and we allow the water to be lost into the air.
Lesson 1: Chemical Changes
A chemical change results in a change in the type of matter present in the object. The resulting substance has different properties from the original matter. If ou leave you bicycle out in the rain throughout summer, it will begin to rust. Rust is a chemical change that occurs on Iron when it is exposed to water and air (oxygen). It appears brown in color and in this case, you cannot reverse the rust beck to iron. Some chemical reactions can be reversed so that you obtain the original substance(s). Most chemical reactions either use up energy, or produce energy. The energy may be in form of heat, light or electricity.

There are very many chemical changes. Here are a few examples:
- Cooking: cooking and baking results in chemical changes on the ingedients.
- When you mix vinegar and baking soda, you see bubbles being released. These bubbles are actually Carbon Dioxide gas being released by the chemical reaction between vinegar and the baking soda.
- Burning paper or wood or other materials also causes chemical changes. The smoke released in a mixture of carbon dioxide, moisture and soot particles. (There are other products too that you dont need to know at this level).
- Rusting
How do you know a chemical change has occured?
There are some tell tale signs that you can use to detect if a chemical reaction has occured. For instance:
- Change in color
- Release of gas
- Smell
- Change in temperature - the substance could either become warmer or colder.
- Sometimes chemical reactions result in production of energy such as light. Fire is good example here.
Another term for a chemical change is chemical reaction. Chemical reactions have two parts. A substance present before a chemical change is a reactant. A substance produced by a chemical change is a product. A chemical equation uses letters and numbers to represent the amounts of reactants and products involved in a chemical change. An arrow separates the reactants on the left from the products on the right.
Lesson 2: Chemical Properties
In previous sections, we learnt that elements have many physical properties such as mass, density, color and conductivity. In this section, we will review the chemical properties of elements.
A chemical property describes the way a substance reacts with other substances. Elements in the same area of the periodic table have similar chemical properties.
Scientists classify metals into 3 categories: Alkali metals, Alkaline metals and transition metals. Alkali metals are located on the far left of the periodic table along with Hydrogen which is not a metal. Alkali metalks include Sodium (Na), Lithium (Li) and Potassium (K). They are soft and extremely reactive, therefore they easily form compounds with other substances. They never exist by themselves in nature.
Alkaline metals are located to the right of alkali metals. These are not as reactive as the alkali metals, but they are also soft and light. They include Calcium (Ca), Magnesium (Mg) etc. They are essential to many living things.
Transition metals are a large group of elements in the center of the periodic table. They include copper, iron, gold, nickel, and zinc. Most transition metals are hard and shiny. They react slowly with other substances. Transition metals are used to make coins, jewelry, machinery, and many other items.
On the right side of the periodic table are metalloids and nonmetals.
Metalloids include silicon, boron, and arsenic. They share properties with both metals and nonmetals. They are semiconductors, i.e., at high temperatures they conduct electricity, like metals, but at very low temperatures they stop electricity from flowing, like nonmetals. Because of this, silicon and other metalloids are used in machinery, computer chips, and circuits. Nonmetals, such as oxygen, carbon, and nitrogen, have properties opposite to those of metals. At room temperature, most of them exist as gases or as brittle solids. Nonmetals cannot be rolled into wires or pounded into thin sheets. Most nonmetals are poor conductors of heat and electricity.
Noble gases, in the far-right column of the periodic table, are nonmetals that do not react naturally with other elements. These gases have many uses. Argon is used in electric light bulbs. Neon, when exposed to electricity, produces the bright colors of some signs. Xenon is used in car headlights. Helium is often used in balloons.
To the left of the noble gases are elements called halogens. These include fluorine and chlorine. They are very reactive nonmetals. Chlorine combines with sodium to form sodium chloride (table salt).

Acids and Bases
Acids: An acid is a substance that:
- Turns blue litmus paper red.
- Acids are sour in taste. Citric acid is what gives lemons a sour taste.
- Acids release hydrogen ions (H+) in solution. The higher the concentration of hydrogen ions, the stronger the acid and the lower its pH.
Strong acids can be very harmful, children should stay away from acids because they can burn your skin.
Bases: Bases are substances that:
- Can turn red litmus paper blue.
- They taste bitter.
- Bases, such as soaps, tend to feel slippery.
- A strong base can be harmful. Such as drain cleaners. Children should not taste any substance that may contain a strong base.
- Bases release hydroxide ions (OH-) in solution. The higher the concentration of hydroxide ions, the stronger the base and the higher its pH.
Water does not change the color of litmus papers. Water is neither an acid nor a base.
Litmus paper and red-cabbage juice are indicators, materials that change color in the presence of acids or bases. Some indicators, such as litmus paper, react either to acids or to bases but not to both.
The pH scale
The pH scale measures the strength of acids and bases. It runs from 0 to 14. A substance with a pH below 7 is acidic, and one with a pH above 7 is basic. A substance with a pH of 7, such as distilled water, is neutral.
Uses of acids and bases
Both acids and bases have many important uses. Strong acids are used in the production of plastics, explosives, and textiles. Sulfuric acid, nitric acid, and hydrochloric acid are all commonly used acids. Strong bases are used in batteries. Ammonia, a common yet strong base, is used in cleaning and bleaching.
Both acids and bases are used by the body. Hydrochloric acid in the stomach kills bacteria and breaks down food during digestion.
Bases are good cleaning agents, because they are slippery and break down grease and oil. Drain cleaners contain bases that are so strong they can even decompose hair.
Salts
Every salt is a compound formed by a reaction between an acid and a base. When an acid and a base are mixed, they react. This process, known as neutralization, produces water and a salt. The hydrogen ions (H+) of the acid and the hydroxide ions (OH-) of the base combine to form water, or H2O. The other atoms of the acid and the base combine to form a salt.
Many salts dissolve easily in liquids. Salts are electrolytes, meaning that they allow an electric current to flow when dissolved in a liquid such as water.
Lesson 3: Carbon and Its Compounds
Carbon is very abundant in many items you see including the graphite used in pencils, coal, diamonds etc. Carbon bonds with itself in several ways. Many carbon compounds are organic compounds, which are the building blocks of living things. Carbon is also present in many inorganic compounds as well, such as Carbon dioxide.
Carbon Dioxide
One molecule of carbon dioxide is composed of one atom of carbon and two atoms of oxygen (hence the di- prefix) - CO2.
Carbon dioxide is a clear, odorless (no smell) gas. It is an inorganic gas but it is closely involved in many life functions. For example, during photosynthesis plants absorb carbon dioxide from the air. Dead organisms release carbon dioxide as they decay. People release carbon dioxide every time they breathe out. Frozen carbon dioxide, (dry ice), is used to keep objects cold. Carbon dioxide is also the gas that is used to make bubbles in a carbonated beverages.
Carbon Monoxide
Carbon monoxide (CO) is composed of one molecule of carbon and one atom of oxygen. It is a clear, odorless gas and forms when fuel does not burn completely (this is called incomplete combustion). It is poisonous to humans and other animals because it prevents blood from carrying oxygen to the rest of the body. Carbon monoxide can be produced by poorly vented gas heaters, fireplaces, and furnaces. People should install carbon monoxide detectors in their homes to warn them if the levels of carbon monoxide increase to harmful levels.
Methane
Methane is composed of one atom of carbon with 4 atoms of hydrogen, CH4. Methane, or natural gas, is used to heat millions of homes throughout the world. Methane is released in marshes. Methane is also a waste product of digestion and is released by animals as a gas. Fertilizers and rubber tires are made using methane-based products. Methane can also be chemically changed into many other useful compounds.
Organic Compounds
Carbon is the most common element of all macromolecules, and therefore carbon is essential to the life processes of all known organisms.
Carbohydrates
Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen. Grain foods such as bread and pasta, as well as sugars, starches, and fruits, are all made of carbohydrates. During cellular respiration, your body produces the energy it needs by turning glucose and many other carbon-based sugars into carbon dioxide and water. This process releases the energy that powers the cells of your body.
Lipids
Lipids include fats, oils, waxes, and cholesterol. They are rich in energy. Lipids can store and release more energy than other organic compounds. Tehy have many carbon-hydrogen bonds, but they have fewer oxygen bonds than are found in carbohydrates.
Proteins
Proteins are important organic compounds in the human body. Proteins consist mainly of carbon, hydrogen, oxygen, and nitrogen. The body uses proteins for several important functions such as:
- Cell growth and repair.
- Move oxygen through the blood.
- Immune-system functions.
- Significant component of the body’s muscles
Foods rich in proteins include eggs, meats, fish, and some vegetables such as peas and beans.
Lesson 4: Atoms and Energy
An atom is a particle that consists of a nucleus, which contains protons and neutrons, surrounded by a cloud of electrons. The atom is the basic particle of the chemical elements. Different elements can be distinguished from each other by the number of protons that are in their atoms.
Atoms are extremely small. A human hair is about a million carbon atoms wide.

More than 99.9% of an atom's mass is in the nucleus. Each proton has a positive electric charge, while each electron has a negative charge, and the neutrons, if present, have no electric charge. If the numbers of protons and electrons are equal, as they normally are, then the atom is electrically neutral. If an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion (or anion). On the contrary, if an atom has more protons than electrons, it has a positive charge, and is called a positive ion (or cation).
Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to attach and detach from each other is responsible for most of the physical changes observed in nature. You will learn more about this in Chemistry.
Radioactivity
Atoms with the same number of protons but different numbers of neutrons are called isotopes.
Some isotopes are unstable, because the atoms have too much nuclear energy. These atoms get rid of their excess energy by giving off invisible rays or particles. A radioactive element gives off energy in the form of rays or particles. Marie and Pierre Curie discovered and named two radioactive elements, polonium and radium. The energy given off by radioactive elements such as these is called radiation.
As an atom gives off its radiation, the nucleus of the atom decays, i.e., it breaks down, into a different chemical element. The amount of time it takes for half of an isotope in a sample of an element to decay (by emitting radiation) is called its half-life. For example, the half-life of uranium 238 is 4.5 billion years.
There are three common forms of radiation.
- Alpha particles are made of two protons and two neutrons. They are relatively large, heavy, and slow. They cannot penetrate many materials.
- Beta particles are electrons. They are fast, light, and able to penetrate some materials.
- Gamma rays are not particles but electromagnetic waves. They have a short wavelength, which means that they have lots of energy. They can penetrate most materials.
Types of nuclear energy
There are two types of nuclear energy:
Nuclear fission is the splitting of a nucleus into two or more pieces when struck with a moving neutron. Nuclear fission produces more free neutrons and releases energy. Remember fission meand 'split' or 'divide'.
Nuclear fusion is another way to release energy. Fusion is to merge nuclei with smaller masses to form one nucleus with a greater mass. During nuclear-fusion reactions, some of the mass of the merging particles disappears. Scientists infer that the missing mass is converted into a large amount of energy. Nuclear-fusion reactions occur only at very high temperatures. In nature, temperatures high enough for nuclear fusion to happen are found in the cores of stars such as the Sun. Fusion reactions produce large amounts of energy in form of heat and light. The energy that is released from fusion reactions is what enables some stars to shine for billions of years.
Uses of radioactivity
Radiation can damage or destroy the genetic information that controls how cells grow and divide. However, radiation is also very useful.
Cancer detection: a radioactive material injected into the bloodstream attaches to cancer cells, and machines show which parts of the body have an increased level of radioactivity.
Radiation can also be used to kill cancer cells. Radiation therapy can damage the cancer cells’ genetic information.
Radioactive materials are also used to produce electricity. Heat energy produced by nuclear fission is used to heat water to run a turbine in order to produce electricity
Lesson 1: Forces and Motion
Everything in the world is in motion, all the time. Even things that look perfectly still are packed with atoms that are vibrating with energy. Motion is caused by forces, for example, if you kick a ball (the force) and it flies into the air (the motion). But forces don't always make things move: a bridge has lots of forces acting on it, but it doesn't move.
Motion can be defined as the change in an object’s position compared to a fixed object. If you ride in a car, your position changes compared to a tree or a telephone pole. An object’s position is its location compared to other objects. In the case of riding a car, your position relative to the car seat, dashboard, or steering wheel doesnt change, so you are motionless inside the car.
Apparent motion is when other objects seem to be moving in the oppposite direction when you are in motion. When you ride in a car, the trees, electric poles and buildings appear to be moving backwards.
Speed, Velocity, and Acceleration
Speed is how fast an object’s position changes with time at any given moment. Speed is calculated by dividing the distance traveled by the time taken to travel.
Speed = Distance ÷ Time
Most moving objects do not travel at the same speed at all times. For this reason, it is useful to calculate an average speed. The average speed of a moving object is the total distance traveled divided by the total amount of time.
Velocity is a description of a moving object’s speed and direction. When you know the velocity and present position of an object, then you should be able to predict where it will be located after a certain amount of time. Any change in the speed or direction of an object causes its velocity to change.
Acceleration is a change in the velocity of an object over time. If the velocity of the moving object increases with time, then the acceleration is in the direction of the velocity. If the velocity of the moving object decreases with time, then the acceleration is in the direction opposite the velocity. Contrary to most people's understanding, an object can accelerate while maintaining the same speed. For example, if a car moved at a constant speed and turned a corner without changing its speed, the change in direction would be a change in the velocity of the car. This means that the car is actually accelerating.
Forces
A force is a push or pull acting upon an object as a result of its interaction with another object. A force constantly applied to an object is called continuous force. A rocket engine provides thrust, which is a strong push in the direction opposite an object’s weight. Thrust causes the rocket to accelerate upward, away from the launch pad. This thrust will continue to be applied as long as the rocket engine burns fuel.
Types of Forces
We have already defined continuous force as that which gets exerted on an object continuously.
Momentary force is the type of force that acts on an object for a very short period of time. It can also be called impact force.
Friction is a force that opposes the motion of an object. Friction occurs when two or more objects come into contact.
Drag force occurs when an object moves through any liquid or any gas, such as air. This force opposes the motion.
Other types of forces will be covered in higher grades.
Friction
Friction is the force that opposes the motion of an object. It occurs when two or more objects come into contact. For example, in order to move a book across a table, you must pull on it with a force that is greater than the force of friction that is reventing the book from moving.
There are many types of friction. For example, the force between the surfaces of two solid objects which keeps the objects from moving is called static friction. The force that opposes the sliding of an object over a surface is called sliding friction. Rolling friction is the force that opposes the motion of a wheel turning along a surface.

Friction is necessary to maintain position and prevent objects from falling. For example, friction is necessary when you want to stop your bicycle or to turn a corner. It prevents the wheels from slipping.
Friction and drag force are similar because both forces oppose motion. However, different types of friction do not depend directly on the size, shape, or speed of a particular moving object. In contrast, all three of these factors do affect drag force. For example, a crumpled piece of paper falls faster than another piece of the same paper that is not crumpled. This occurs because of the way that air affects differently shaped objects.
Net force is the sum of all the forces that are acting on an object. When the net forces are equal in strength and opposite in direction, they are balanced forces. The motion of an object remains unchanged. Forces of unequal strength or forces that are not opposite in direction are called unbalanced forces.

Balanced force

Unbalanced force
Newton's Laws of Motion
Newton's First Law
Newton’s first law of motion states that an object at rest tends to stay at rest and that an object in motion will remain in motion unless an external force is applied on the object. In other words, an object moving in a straight line at a constant speed tends to keep moving that way. The tendency of an object to keep moving at the same speed and in the same direction is called inertia.
Picture a ball resting in the aisle of a bus that is moving at a constant velocity. When the bus slows down, the ball rolls toward the driver. The bus and the ball moved at the same velocity. The bus would have continued to stay in motion, but the brakes provided a force that changed its velocity. However, the ball was not attached to the bus, so it stays in motion.
Lesson 2: Changes in Motion
Newton's Second Law of Motion
Newton's second law states that Acceleration depends on the object’s mass and the amount of net force applied to it. Newton’s second law can be written as a formula:
a = F ÷ m
An object’s acceleration (a) equals the net force on the object (F) divided by its mass (m). If the force increases, then the acceleration also increases. However, if the mass increases, then the acceleration decreases.
The combination of the mass and the speed of an object is called momentum. A baseball has more momentum than a tennis ball traveling at the same speed because the baseball has more mass. A tennis ball can have more momentum than a baseball, if the tennis ball’s speed is great enough.
Momentum is useful for studying the motion of colliding objects. Total momentum does not change when objects collide. Scientists call this principle conservation of momentum.
Newton's Third Law of Motion states that for every action (force) in nature there is an equal and opposite reaction. If object A exerts a force on object B (action force), object B also exerts an equal and opposite force on object A (reaction force). In other words, forces result from interactions.
Newton’s third law of motion explains how rockets lift off. Burning fuel produces hot gases, which are pushed downward from the rear of the rocket. The force of the rocket on the gases is the action force. The reaction force is the upward force exerted by the hot gases on the rocket.
Newton's law of gravitation, states that any particle of matter in the universe attracts any other with a force varying directly as the product of the masses and inversely as the square of the distance between them. According to this law, the planets, the stars, and all particles of matter exert gravitational force. Newton’s explanation changed the ways in which scientists viewed the solar system. This explains the Moon’s orbit around Earth and Earth’s orbit around the Sun.
Weightlessness
Weightlessness is the state of being without detectable weight. Astronauts on the International Space Station are in free fall all the time. Gravity still pulls on the Station, but because it travels around the Earth at such high speed, its travelling forwards equals out the falling and the ISS stays more or less at the same height. The astronauts inside it experience weightlessness, floating around in no particular direction. There's no up or down for them... down is wherever their feet are.
Lesson 3: Work and Energy
In Science, work is defined as the force to move an object through a distance. Based on this definition, holding a heavy box in the same position results in no work being done because the box did not move.
Work is equal to the force of a push or pull multiplied by the distance the object is moved. The force must act in the same direction as the motion. If the force is expressed in newtons and the distance is expressed in meters, the units for the work done are newtonmeters (Nm), also called joules (J).
Suppose you use a rope to lift a bucket filled with rocks up to a tree house that is 5 meters high. The weight of the bucket is 30 newtons. You can calculate the work done by using the formula:
Work = Force X Distance
Work = 30 N X 5 m
Work = 150 Nm also = 150 J
Energy is the ability to do work. Like work, energy is measured in joules. There are several forms of energy. For example, an object placed on an elevated position stores energy because of its position and the force of gravity. This energy is called Potential energy. An object that is in motion also carries energy called Kinetic energy. This is due to the obect's mass and speed.
Energy usually changes from one form to another. When an object is moving uphill, it slows down, which means it loses kinetic energy, but because its moving to a higher elevation, it gains potential energy. when the object begins to move downhill, it gains more kinetic energy and loses potential energy.
Thermal energy is the heat energy in an object.
Conservation of Energy
Energy cannot be created or destroyed, however, it can change from one form to another. All forms of energy have a source, a means of transfer, and a receiver. For example, in a flashlight the energy source is the potential energy in the battery. An electrical circuit enables the energy to be transferred to the bulb. The bulb is the receiver of this energy. It can then give off energy in the form of light and heat.
The following are examples of various forms of energy:
- Nuclear energy - radioacive material, the sun.
- Electrical energy - battery
- Light energy - lump, the sun, flashlight.
- Chemical energy - food
- Mechanical energy - Moving parts of a machine
- Sound energy - Speakers
- Thermal energy - hot water, the sun
Changing Forms of Energy
Several devices convert one form of energy to another.
A hair dryer converts electrical energy into thermal energy
A speaker converts electrical energy into sound energy
A microphone converts sound energy into electrical energy
A bulb converts electrical energy into light energy (and some thermal energy).
Light energy from the sun is converted into chemical energy by the green leaves of trees through photosynthesis. This chemical energy can be transferred from plants to animals through ingestion. Some parts of the animals will convert this chemical energy into mechanical energy (muscles), thermal energy (for homeostasis), electrical energy (neurons) and sound energy (mouth).
Power
Power is the amount of work done per unit of time, i.e., work divided by time. Because work is expressed in Joules, and time is expressed in seconds, power is expressed in J/s. One J/s is also known as a watt (W). The watt is the standard unit of power.
Light-bulbs indicate the power they use per second. The more power a light bulb has, the brighter the light bulb is. Household bulbs have about 100 watts or less.
The prefix kilo- means 'one thousand' so a kilowatt is equal to 1,000 watts.
Horsepower (hp) is a measurement that was described over 200 years ago when James Watt wanted to compare the work done by a device to that done by a horse. 1 horsepower equals 746 watts. As you can tell, the watt is named in honor of James Watt.
Lesson 4: How Machines Work
A simple machine is a device with few, if any, moving parts that makes it easier to do work. For example, a hammer is a simple machine, it doesnt have parts but it makes it easier to remove a nail. Simple machines change either the force required, the direction of the force, or the distance through which the force is applied. The hammer, for example, remove nails by changing the direction of the force applied and also increasing the strength of the force applied. It changes the direction of the force because we push down the handleof the hammer and the hammer pulls the nail upward.
The force you apply to a simple machine is called the Effort. (or effort force). The force against which the machine acts is called the resistance force. The force that the machine applies to an object as a result of the effort force is called the output force.
The number of times a machine multiples/amplifies the effort force is called the Mechanical advantage. (MA) The MA of a machine is equal to Output force ÷ Effort force. Suppose you applied a force of 100 newtons to a simple machine to lift a box that weighed 500 newtons. The mechanical advantage would be 500 ÷ 100 = 5. This means that the machine would multiply your effort force by 5.
Types of Simple Machines
There are two main classes of simple machines: the lever and the inclined plane. The lever class also includes the wheel and axle and the pulley. The inclined-plane class also includes the wedge and the screw.

Levers
A lever is a simple machine consisting of a rigid barand a pivot point. The pivot is called a Fulcrum. The part of the bar on which a person applies an effort force is called the effort arm. The portion of the bar on which the lever produces an output force is called the resistance arm.

The positions of the fulcrum, effort force, and output force vary among levers. Based on these differences, there are three classes of levers.

First-Class Levers
In a first-class lever, the fulcrum is between the effort force and the output force. Therefore, a first class lever changes the direction of the effort force. The output force is greater than the effort force when the fulcrum is closer to the output force than to the effort force—that is, when the effort arm is longer than the resistance arm. The mechanical advantage can be calculated by dividing the distance the effort arm moves by the distance the resistance arm moves OR by dividing the length of the effort-arm by length of the resistance. A see-saw is an example of a first-class lever.
Second-Class Levers
In a second-class lever, the output force is between the effort force and the fulcrum. Second-class levers do not change the direction of the effort force. However, they produce a mechanical advantage because the effort arm is longer than the resistance arm. A wheelbarrow is an example of a second-class lever.


Third-class lever
In a third-class lever, the effort force is between the output force and the fulcrum. Like second-class levers, third-class levers do not change the direction of the effort force. But, unlike second-class levers, third-class levers always produce an output force that is less than the effort force. A fishing roda and the hand muscles are examples of third-class levers. A third-class lever multiplies the distance of the effort. A person would only need to move her hands a short distance to move the tip of the rod through a greater distance.


Wheel and Axle
The wheel and axle is a type of a first-class lever simple machine. There is a wheel that applies the effort force and a smaller axle that produces the output force. The mechanical advantage of a wheel and axle is calculated by dividing the length of the effort arm by the length of the resistance arm. The effort arm is the radius of the wheel. The resistance arm is the radius of the axle. Since the effort arm can be quite large compared to the resistance arm, this machine can have a large mechanical advantage.
Examples of wheel and axle machines



Pulley
Pulley is a grooved wheel that turns by the action of a rope in the groove. When the rope moves, the wheel turns. A pulley is also a type of lever, one in which the rope forms the arms and the wheel serves as the fulcrum.
A pulley may be either fixed or movable. A fixed pulley makes work easier by changing the direction of the effort force. It does not change the strength of the effort force itself.

The wheel of a movable pulley is attached to the object being lifted and moves with it. A single movable pulley multiplies the effort force by 2, so it has a mechanical advantage of 2. However, a single movable pulley does not change the direction of the effort.

A pulley system is made up of several pulleys acting together. Some pulley systems contain both fixed and movable pulleys. The addition of a fixed pulley enables the system to change the direction of the effort. The mechanical advantage of a pulley system can be expressed in terms of the distance it moves an object compared to the distance its rope must be pulled when the effort is applied. This can be done by by dividing the distance the effort rope moves by the distance the object moves. A simple way to measure the mechanical advantage of a pulley system is to count the number of rope strands pulled downward by the object being lifted. This number is the mechanical advantage of the system.

Inclined Planes
An inclined plane is a straight slanted surface that can multiply an effort force. It makes it easier to move a heavier load upward.
The mechanical advantage is equal to the output force divided by the effort force. Suppose two students use ramps to slide boxes weighing 300 newtons onto a stage. One student uses a steeper, shorter ramp and applies an effort force of 225 newtons. The other uses a shallower, longer ramp and applies an effort force of 135 newtons. The effort force of the steeper ramp is 225 newtons. Its mechanical advantage is 300 divided by 225, which equals 1.33. The effort force of the longer ramp is 135 newtons. Its mechanical advantage is 300 divided by 135, which equals 2.22. The longer ramp has the greater mechanical advantage. The mechanical advantage can also be calculated by dividing the length of the incline by the height.

Screw
A screw is another simple machine. The spiral ridges called threads move into an object as the head of the screw turns. The space between the threads is called the pitch. A screw’s mechanical advantage is calculated in a similar way to a ramp’s. If the distance around the head of a screw were 1.5 centimeters and its threads were 0.1 centimeters apart, its mechanical advantage would be 1.5 divided by 0.1, which equals 15.

Wedges
A wedge is an inclined plane that changes the direction of an applied effort force. A knife is a wedge. When you push down on a knife to cut food, the knife presses sideways against the food, pushing it apart. A wedge may be a single inclined plane or two inclined planes joined back-to-back. Wedges that are thin have greater mechanical advantages than those that are thick.

Compound Machines
A compound machine is a combination of two or more simple machines. For example, scissors include two levers and two wedges. The pivot point for the blades and handles is the fulcrum, and the blades are the wedges. A bicycle is also a compound machine. The pedals and wheel and axle machines. The gears are also wheel and axle machines. The brakes work as two levers.
The work put into a machine is always greater than its resulting work output because friction causes some of the work input to be lost usually as heat. The wasted energy reduces a machines efficiency. Efficiency is the ratio of the work done by a machine to the work that was put into it. To calculate efficiency, divide the output work by the effort work. Coating certain parts of a machine with substances such as oil can reduce friction thereby increase efficiency of a machine.
Lesson 1: Waves and Sound
A wave is a disturbance that transfers energy from one point to another. Some waves, such as light waves, can travel through empty space. While other kinds of waves, such as sound waves, must travel through a medium. A medium is a substance that can transfer energy. A medium can be a solid, a liquid, or a gas. The movement of particles by a wave is called vibration.

Waves are classified by the type of vibration they cause in a medium. For example, waves that cause matter to moves up and down are called Transverse waves. Waves that cause matter to move back and forth are called compressional waves. The vibration of the coils produces a compression, an area where particles are pushed together. Behind the compression is a rarefaction


Parts of a wave
On a transverse wave, a crest is the highest point. The crest in a compressional wave is the point with the greatest compression. A trough is the lowest point of a transverse wave. On a compressional wave, a trough is the area with the greatest rarefaction.
Wavelength is the distance between wave crests or troughs. Frequency is a measure of how many wave crests or troughs pass a given point in one unit of time, such as a second. High-frequency waves have shorter wavelengths and transfer greater energy. The period of a wave is the amount of time it takes for a wave to complete one full cycle. Period is the inverse of frequency. Amplitude, the height of the wave from its trough or crest to its midpoint, is a measure of the wave’s intensity. In the ocean, a wave's amplitude increases as it nears the shore. Frequency is measured in hertz (Hz), the number of waves per second. Hertz means 'cycles per second' with respect to frequency.



Factors Affecting Wave Speed
The medium through which the wave travels can affect the speed. The deeper the water, the faster the wave travels. Sound waves move faster through solids than liquids and even slowest in gases.
A sound wave is a compressional wave produced by the vibrations in the matter. Molecules in the medium move back and forth, pushing nearby molecules. A compression forms as the molecules are pushed closer together, and a rarefaction occurs behind the compression. For this reason, sound waves cannot travel through empty space. Tuning forks vibrate at particular frequencies which procude compressions and rarefactions in the air.
Sound Reflection
Sound reflection occurs when sound waves bounce off an object. Reflected sound is called an echo.
A technology called sonar uses reflected sound waves, or echoes, to locate unseen objects and to make maps of the ocean floor. Sonar works by bouncing sound waves off an object and measuring the time the echoes take to return.
Sound Refraction
Refraction occurs when the direction of a wave changes because of a change in the medium. For example, sound waves traveling through air will be refracted as they enter water. This change in medium also changes the waves speed.
Absoprtion of Sound Waves
Hard surfaces easily reflect sound. Soft surfaces absord sound waves. For example, people add insulation to walls and use soft ceiling tiles as a way to absorb sound in offices, libraries, movie theaters etc.
The Doppler Effect
Doppler effect occurs when the pitch of sound seems to change because the source of the sound or the listener is moving. This is what happens if you are watching racing cars and the frequency of their sound increases to a high pitch when the car gets closest to you then reduces again as the car moves away.
Try it here with sound onVolume of a Sound
The loudness of sound is called volume. The amount of energy or intensity determines the volume of the sound. The volume of sound is measured in decibels (dB). A whisper has a volume of about 30dB. Regular speech is around 80dB. Sounds hire than 90dB can damage a person's hearing.
Interference
The presence of two or sound waves passing the same medium at the same time is called interference. Interference can be positive or negative. If the crests or troughs of the waves meet, the combined sound waves would produce a louder sound than that produced by one speaker. This is called constructive interference. If the crest of one wave meets the trough of the other wave, the sound waves together have a lower amplitude than the sound made by one wave alone. This is called destructive interference.
Hearing

When sound waves reach the ear, they pass through the ear canal to the eardrum. The sound waves strike the eardrum and cause it to vibrate. The vibrations stimulate nerve cells located deep inside the ear. These vibrations are then converted to nerve impulses that your brain recognizes. By interpreting pitch and sound quality, the brain identifies different sounds.
'Music' is a combination of sounds that a listener finds pleasing, while 'noise' is a combination of sounds that a listener finds unpleasant. Sound that is interpreted as musical has a mathematical structure of both tones and silence. This structure is often referred to as rhythm. Sounds that lack harmony, rhythm, and mathematical structure are interpreted as noise.
Lesson 2: Properties of Light
Light is a form of energy that travels in waves. Light waves spread out as they move away from a source. Light travels in straight lines called rays. Light waves can travel through empty space, without needing a solid, liquid, or gas medium. Light travels through space at about 300,000 kilometers per second, which is the fastest speed matter and energy can possibly reach. Natural sources of light include the Sun (and other stars), and lightning. Human-made light sources, such as lamps and candles, rely on chemical reactions or electricity to produce light. Light rays from any source always travel in straight lines. A light wave will spread out if it travels past the edge of a thin object or if it moves through a narrow opening. Regardless of its source, a ray of light will not change direction unless it travels through a different medium or is disturbed in some way.
Light interaction with matter
Different kids of matter will interact with light differently. Transparent matter allows light to pass through with almost no disturbance. Translucent matter allows some light to pass through while some light is either blocked or bent. Objects seen through a translucent material do not look crisp clear, they appear blurred. Opaque materials reflect or absorb all light. Usually the absorbed light is converted to heat energy. If you look through an opaque material, you cannot see through. A shadow is a dark area produced by an opaque object blocking light from passing through.
Light reflection
Reflection occurs when waves bounce off a surface. The angle between an incoming light ray and the reflected ray to the surface are the same.


A mirror is an object with a polished surface that forms reflected images. The things you see in a flat mirror look almost as if they exist on the other side of the mirror, with one important exception. The image that appears in the mirror is reversed.
The shape of a mirror affects the appearance of the image it reflects. Most everyday mirrors are plane/flat mirrors. They have a flat surface and images appear as exact copies, though they are reversed.
Concave mirrors have a surface that curves inward. Light rays are reflected from the surface of a concave mirror and meet at a point located in front of the mirror. An object you placed close to a concave mirror would produce a large image that was right-side-up. As you moved the object away, the image would become blurry and eventually appear upside down. The image would stay upside down and become smaller as you move the object farther away from the mirror. Makeup and shaving mirrors are often concave, because they make the face appear larger and allow people to see greater detail.


Convex mirrors have a surface that curves outward. A convex mirror produces an image that is right-side-up and much smaller than the object. This wide-angle view makes convex mirrors useful for security in stores and also for providing a better view for drivers of vehicles.

Lenses
Convex lenses form images by refracting light rays together. A convex lens is thicker toward its middle, and this gives the lens a shape that bulges outward. Light rays pass through the lens and come together at a point on the other side. The focal point is the point at which the light rays meet. The distance between a convex lens and an object determines the type of image that forms. If the object is located between the lens and its focal point, the image that is formed is right-sideup and larger than the actual object. If the object is located beyond the focal point of the lens, the image that is formed is upside down and smaller than the actual object.
A concave lens curves inward. This type of lens forms an image by spreading light rays apart. A concave lens is thinner in the middle than it is at the edges. An image that is formed by a concave lens is right-side-up and smaller than the actual object. Concave lenses are used mostly in eyeglasses to correct nearsighted vision.
Vision Impairment
A nearsighted person has at least one eye that is longer than normal from front to back. This causes light rays from distant objects to be focused in front of the retina. As a result, nearby objects appear clear, but distant objects look blurry. Eyeglasses or contact lenses with concave lenses correct nearsighted vision.
A farsighted person has at least one eye that is shorter than normal from front to back. This causes light rays from nearby objects to be focused behind the retina. A farsighted person can see distant objects clearly but has difficulty viewing nearby objects. Eyeglasses or contact lenses with convex lenses correct this condition.

Lesson 3: Light Waves and Color
Visible light contains a mixture of wavelengths that the human eye can detect. When these wavelengths are separated, we see them as different colors.This separation occurs de to refraction. Different wavelengths are refracted in different amounts. Long, red wavelengths are bent the least, and short, violet wavelengths are bent the most. Recombining all the wavelengths of visible light produces white light.
A prism is a triangular piece of glass or plastic that can bend light. This refraction separates visible light into red, orange, yellow, green, blue, and violet. Light can also bend by using diffraction grating. This concept was first described by Sir Isaac Newton in he late 1600s by observing that sunlight passing through a prism emerged as bands of different colors. Newton hypothesized that sunlight was naturally made of different colors of light. He called these colors a spectrum.
Sunlight striking an object may be reflected, refracted, or absorbed. The light that is reflected determines the color of an object. For example, when sunlight strikes a leaf, many wavelengths are absorbed and used in photosynthesis. Green light is reflected, so the leaf appears green. An object that reflects all visible light appears white. An object that absorbs all visible light appears black.
Visible light makes up only a small portion of these waves. The electromagnetic spectrum contains the full range of wavelengths. The spectrum is arranged from long waves, with the lowest amount of energy, to short waves, with the highest amount of energy. It consists of radio waves, microwaves, infrared waves, visible light, ultraviolet rays, X rays, and gamma rays. Radio waves have the longest wavelengths and include transmissions of AM radio, shortwave radio, television, and FM radio. Ultraviolet, or UV, rays carry more energy than visiblelight waves do. Overexposure to ultraviolet rays and other high-energy waves can damage people’s skin and eyes. X rays can penetrate many substances, including soft tissue is humans and animals. Because of this property, X rays are used to make images of hard parts of the body, such as teeth and bones. Gamma rays have very short wavelengths and have so much energy that they can even pass through some metals and concrete.

Mixing Colors
Colors can be grouped into Primary or Secondary colors. Primary colors are not produced through any mixing process. Secondary colors are produced by blending primary colors. Primary colors include Red, Green and Blue (RGB). In the RGB (red, green, blue) color model, primary colors of light combine and produce almost all colors. The RGB color model is an example of additive color mixing. In this color model, the three primary colors can combine, reflect all colors, and produce white.
Color printers have ink cartridges containing cyan, magenta, and yellow (CMY) pigments. This CMY model uses subtractive color mixing. The perceived color depends on the ability of the substance’s pigments to absorb wavelengths of light. When all three pigments combine, this produces black.


RGB and CMY color models
Lesson 4: Heat
Heat is the flow of energy from one substance to another. Heat is in the form of kinetic energy caused by the movement of the molecules that make up all matter. If you warm something, such as a pot of water, you increase the movement of water molecules and the substance then becomes hotter. As an object is heated, the total amount of kinetic heat energy, or thermal energy, within that object increases.
The metric unit used to measure this energy is the calorie. A calorie is the amount of energy needed to raise the temperature of 1 gram of water by 1oC.
Temperature is a measurement of the average kinetic energy of the molecules in a substance. As heat flows into a substance, the kinetic energy of the molecules in the substance increases. This causes an increase in temperature.
This increase in energy can also cause molecules to move farther apart. As the molecules in a substance spread out, it usually increases in volume. An increase in volume that is caused by an increase in temperature is called thermal expansion.
The total amount of thermal energy in a substance depends on temperature and mass. Overall there is more energy in a gallon of warm water than there is in a thimble of boiling water. Although the temperature of the jug is lower, the total amount of energy is much higher.
How Heat Travels
Heat energy always flows from a higher-temperature material to a lower temperature material until an equilibrium is established where the temperatures of both materials are the same.
Heat energy can move in three ways:
- Conduction: is the movement of energy through direct contact. The energy flows directly from one material to the other. Conduction is the only way that heat energy can travel through solids. Insulation is any material used to prevent heat from flowing into or out of a substance.
- Convection: When you boil a pot of water, the hot water located at the bottom of the pot rises to the top and the cooler water at the top of the pot moves to the bottom. Convection describes this movement of liquids or gases but not solids. These particles move faster and farther apart.
- Radiation: Radiation is the transfer of energy without any movement of matter. Energy that is transferred in this way is called radiant energy or electromagnetic radiation. This is how the energy from the sun reaches the earth. Objects that absorb radiation gain energy. Objects that are near or below room temperature give off infrared radiation. When objects are heated to about 600°C, they give off a great deal of visible light. This visible light is observed as a dull red glow, like that of a stove-top burner.
Body heat
During cold weather, the body attempts to preserve as much heat as possible and prevent heat loss to the environment. This can be achieved by wearing thick clothes made from materials that are good insulators such as wool. Because black absorbs more heat, most winter clothes are also dark colored. In summaer, the body needs to dissipate more heat. So summer clothes are usually light, birghtly colored and allow easy flow of air to enhance evaporation.
Home Heating Systems
The most common heating systems either heat air and circulate it throughout the house in a forced-air heating system, or use hot water that is pumped through pipes into a radiator where the cold air around the radiator is heated and circulates in the room by convection.
Combustion Engines
Gasoline is the fuel that is burned in the engine of a car, but heat energy actually makes the engine move. Heat causes the gases from the burning fuel to expand. The gases push on pistons, which then move downward. The motion of the pistons triggers a chain of actions that turns the crankshaft and propels the vehicle forward. Coolant in the engine's radiator prevents overheating.
Temperature
Temperature is a measure the average kinetic energy of a substances molecules. Some thermometers are made of a clear tube containing a liquid that expands when it warms. Others are made of coiled bimetallic strips that expand from absorbing heat energy. Many modern thermometers are digital.
Specific Heat
The specific heat of a substance is the amount of energy, often measured in joules (J), needed to raise the temperature of 1 gram of the substance by 1°C. Most metals have a low specific heat, so little energy is needed to increase their temperatures. Water has a high specific heat, so more energy is needed to raise its temperature.
Lesson 5: Electricity and Magnetism
Remember, all matter is made up of atoms, and all atoms are made up of protons, neutrons and electrons. The protons are the positively charged particles and the electrons are the negatively charged particles. When the positive and negative particles are equal, the charge equals out. When there are more electrons than protons, you have a charged atom, called an ion.
Electrons spin around the outside of the nucleus and are held in that place by the force of attraction from the protons in the nucleus. However, electrons can be lost by one atom and picked up by another atom resulting in a change in the charge of both atoms. The atom that has lost the electron now becomes positively changred (because it has more protons than electrons) and the atom that gains an electron becomes negatively charged, because it now has more electrons than protons. The transfer of charged particles from one atom to the other can build a series of electrically charged atoms. Electricity refers to the movement and transfer of the energy of charged particles. This energy is used to power motors, lights, appliances, and many other devices.
Static Electricity
When two materials touch one another, electrons can move from one material to the other. This causes one maerial to become more negatively charged and the other positively charged. This transfer of electrons causes an imbalance which results in static electricity. Objects with the same electric charge repel each other and objects with opposite charge attract each other. Some materials lose electrons more easily than others while others attract electrons more easily. For example, the atoms on the human skin more readily lose electrons, becoming positively charged. The atoms on a cat's fur do not lose electrons easily, so if you pet a cat you can create static electricity.
When a charged object is placed near a neutral object, the charged object can affect the overall charge of the neutral object. Like charges within the neutral object are repelled, and unlike charges are pulled toward it. This movement can result in an induced charge. An induced charge is a static charge caused by the presence of an object that itself has a net positive or negative charge. When you rub a balloon on your hair, some electrons leave your hair and are transferred to the balloon. The balloon then has a net negative charge. When you place that balloon near a wall with no net charge, the negative particles in that area of the wall are repelled. This leaves a net positive charge on the surface of the wall. The balloon and wall then attract each other, and the balloon sticks to the wall. Evidence indicates that lightning can also be produced as a result of induced charges. Storm clouds can accumulate a negative charge near the bottom of the cloud. This can induce a positive charge in the ground below the cloud. This imbalance of charges can result in the discharge called lightning, which can reach 5 km in length.
Condutors and Insulators
A conductor is a material through which an electric charge flows easily. Most conductors are made of atoms from which some electrons are likely to become unattached. Metals such as copper are the best conductors. This is why copper is commonly used in electric wiring.
An insulator is a material that does not allow an electric charge to transfer easily. Conductors and insulators of electric charge are very similar to conductors and insulators of heat energy.
If a bare wire touched the metal case of an appliance, it could become electrified and can harm people when they touch the appliance. To avoid this problem, a grounding wire is connected to the metal case of the appliance. The grounding wire connects the case to the ground and because this charge gets distributed over much of Earth, the charge on the case is then too small to cause problems.
Circuits
Electric charges flow through conductors along different paths. Each path for electric charge is an example of a circuit. In circuits, electric charges move within wires, bulbs and other devices.
A simple circuit consists of an energy source such as a battery, a device such as a lamp, and connecting wires. The flow of an electric charge through a circuit is called current electricity. In a circuit, energy from a source such as a battery causes an electric charge to flow through the wire. Electrons that are not strongly attached to the atoms inside the wire move, causing current electricity.
Batteries stop working when the chemical reactions inside them can no longer transfer energy to electrons and move them through the wire in this manner.
Although the movement of negatively charged electrons is most often referred to when studying current electricity in wires, current is always said to flow from the positive to the negative terminal in a circuit. This is called conventional current. This way of describing the movement of electric current originated before scientists fully understood electricity. However, it is still the way used to describe how circuits operate.
A switch can control the flow of a charge in a circuit. When the switch is opened, the flow is halted. The circuit is incomplete and is then called an open circuit. When the switch is closed, the electric charge resumes its motion. When current flows once again, the circuit is called a closed circuit.
Direct and Alternating Current
Direct current, or DC, refers to current that flows in one direction. Batteries provide DC, as do solar-powered cells. The very first commercial electric power stations also used DC.
Alternating current, or AC, refers to the electric charge that does not flow through the circuit in one direction. AC power is transmitted when the charge changes direction, moving back and forth at regular intervals. The main advantage of AC is that this type of current can be transported over long distances with far greater efficiency.
Resistors
Resistors lower the amount of electric charge that flows through a device. Lights and other devices connected in a circuit act as resistors, because they too reduce current flow. A light bulb converts electrical energy to both heat and light.
Types of Circuits
In a series circuit, there is only one path along which current electricity can flow. Each battery supplies more energy that causes electric charge to flow. The light bulbs receive the sum of the energy that comes from the two batteries. This energy is measured in volts (V). Because each battery has 1.5V, the two batteries together deliver a total of 3V. A higher voltage causes more electric charge to move through the light bulbs.
The total resistance is the sum of resistances of the individual devices such as bulbs. For example, two identical light bulbs together in a series circuit will have twice the resistance of either bulb by itself. The voltage of current electricity from a normal wall outlet is about 120V. Each small bulb on a strand of lights operates on about 2.5V. This means that the voltage from a wall socket is about 50 times the voltage that a single bulb requires. To provide the correct voltage to each bulb, each strand could only have 50 bulbs. If there were more, then each bulb would not receive enough voltage to light up.
Parallel Circuits
In a parallel circuit, there are multiple paths along which current electricity can flow. For example, in a string of lights wired in a parallel circuit, when one bulb burns out, there are other paths along which electric charge can flow to all the other bulbs. Parallel circuits are used everywhere that we use electricity, including homes, stores, and offices. If any one device on the circuit burns out, the other devices on the circuit will keep working.
Short Circuits
A short circuit is a path for current electricity that has little or no resistance. Current flowing in a short circuit can reach dangerously high levels and will also generate heat. A fuse is a device that prevents dangerous levels of current from continuing to flow through a circuit. A fuse contains a piece of metal that melts if it is heated. This melting breaks, or opens, the overloaded circuit.
Magnetism
Magnetism is the ability of an object to push or pull another object that has magnetic property. Magnets also work with metals like iron and nickel.
A magnet has two poles, North (N) and South (S). Like poles repel, while unlike poles attract. Magnets always have a N and S pole, even if you break a magnet into two, it will form 2 magnets, each with a N pole and a S pole.
The Earth acts as a magnet with a North and South pole.

Atoms also act like magnets. In most non-magnetic materials, the N and S poles are random so they cancel each other. In materials where the N ans S poles of the atoms are aligned in the same direction, they form a permanent magnet. Iron, Nickel and Cobalt are attracted to magnets because their atoms can align to match those in the magnet thereby acting as weak magnets. When you place pieces of these metals over a magnet they form lines which correspond to the forces around a magnet called magnet field. The closer the lines are, the stronger the magnetic force in that area.

Electromagnets
An electromagnet is an electric circuit that produces a magnetic force. The moving electrons in the circuit generate a magnetic field.
The simplest electromagnet is a straight wire. The magnetic field circles around the wire when there is current flowing through the wire.
When you use the wire to make a loop, you increase the magnetic force. Many loops together result into a coil, which has a much stronger magnetic force.
If you place an iron rod in the coil, it becomes magnetized and makes the eletromagnet even stronger.
A generator is a device that generates an electric current by spinning an electric coil between the poles of a magnet. Energy is used to rotate the axle then as the coil moves through the magnetic field, the margentic forces push on its electrons and generate an electric current. Whenever the coil passes the pole of a magnet the direction of the current changes. This kind of current that regularly changes direction is called alternating current (AC). In US, generators produce alternating current that changes direction 120 times every second.
Current electricity is first conducted to a transmission substation. The substation has many towers with power lines leaving them. Step-up transformers in the substation increase the voltage of the current electricity. This allows the electric charge to be transmitted over long distances more efficiently.
The high voltages that are used in transmitting current electricity over distances are dangerous. Therefore, before current electricity enters your home, the voltage is decreased at several stages. Transformers are again used to change the voltage of the current electricity. Step-down transformers decrease the voltage of the electric charge. By the time the electric current reaches your home, the voltage is 240V. A regular wall socket in your home delivers 120V, and this is what most appliances need to operate. Sockets that deliver higher voltage are used for specially designed appliances, such as certain types of clothes dryers, stoves/ovens or air conditioners.
Saving Energy
You can save fuel and save money by conserving energy. Wearing warm clothes instead of turning up the heat saves energy. Opening a window instead of using a fan saves energy. Turning off lights and appliances when they are not in use also saves energy.