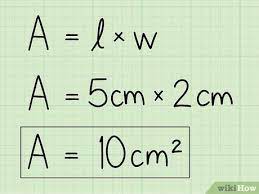Lesson 2: Measurement
Measuring is one way to determine the size of an object, and to compare it with the size of another object. For this to be successful, we need to use the same units of measurement so that different people can understand what the measurement actually means. These units that people agree on are called standard units. Scientists use the metric system which uses prefixes like milli-, centi -, and kilo- . The metric system is based on units of ten to move from one prefix to the next. For example, 1 centimeter is made up of 10 millimeters. 1 meter is made up of 100 centimeters, and 1 kilometer is made up of 1000 meters.
Length: Length is the measure from one end to the other end. It is usually the measure of the long side of a 2D shape. For example, if you place your book on the desk, the length is the measure of the long side of the book.
Width: Width is the measure of how wide an object is. It corresponds to the shorter side of a 2D shape.
Area: Area describes the number of unit squares that an object's surface covers. An easy way to calculate the area of a rectangle is to multiply the Length (L) and the Width (W). Therefore, A = L X W. If the length is 15 centimeters (cm) and the width is 10 cm, the Area is = 15 cm X 10cm = 150 cm2. cm2 is read as 'square cm. When we express Area as A = L X W, we call this a formula. So the formula to calculate the area (A) of a rectangle is L X W. This formula remains the same if we are calculating the area of a square. However, because the square has equal sides, the measure of L will be equal to the measure of W, so even if you switch L and W, you still get the same correct answer. A square with sides equal to 10 cm has an area of L X W = 10cm X 10cm = 100 cm2

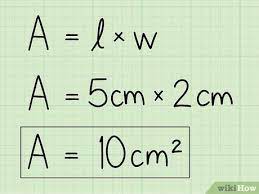
Volume: Volume describes the number of cubes that can fit inside an object. It is a measure of how much space an object occupies. Therefore the object needs to have a 3 dimensional structure. Here, we introduce the concept of height (H). If you are measuring the volume of an ice cube that measures 2cm on all sides, the volume is; V = L X W X H = 2cm X 2cm X 2cm = 8 cm 3. Realise that the formula to calculate the volume of an object can be shortened to V = A (of the base) X H or short V = Base X height. This is important because the same formula applies if the object is based on rectangular sides or if the base is of a different shape. A 3D shape with a circular base is called a cylinder.
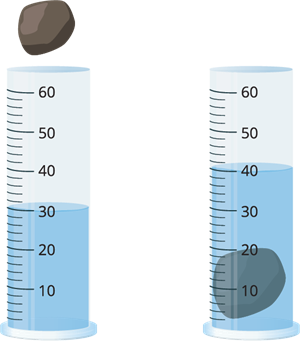
To find the volume of an irregularly shaped object, you can use water in a measuring container. First add the water, measure how much water is in the container. Then surmerge the object until it has all been covered by the water then record the new reading of the volume of water. The volume of the object is the difference between the new final reading and the original reading. This is called displacement. A solid object will displace an amount of water that is the same as its own volume.
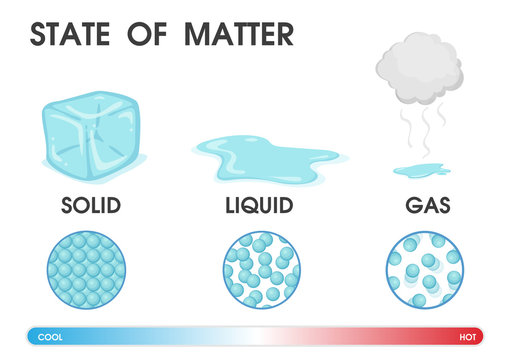
Density: Density is the amount of matter per unit space (volume). Remember we said solids have particles that are tightly packed and gases have particles that are far apart, this corresponds with how much matter is in a unit volume, so solids are expected to have more matter per unit volume than gases. This also means solids are expected to have a higher density than liquids, and liquids have a higher density than gases. in addition to this, objects of the same state can have different densities. As in, different solids can have different densities. The amount of matter is measured using Mass and is expressed in grams (g). To calculate the density of an object, divide its mass by its volume, M ÷ V. If the mass is in grams and the volume in cubic centimeters, then the result will have units in grams per cubic centimeter (g/cm3).
Density and Bouyancy: Remember, bouyancy is the upward force of a liquid or gas on another object. It determines if an object will sink or float in the liquid, such as water. An object floats if the its density is lower than the density of the liquid. For example, the Density of water is 1g/cm3. The density of cork is 0.24g/cm3. Will cork float or sink? Cork will float, because water has a higher density than cork. Brass has a density of 8.5g/cm3. Will brass float or sink in water?
Liquids can also float on top of water as you observe when you add oil into water.
You can change the density of an substance by either heating it or cooling it. Heating makes the particles move faster, making the particles move further apart and this reduces the object's density. This is the reason a hot air balloon will rise up in the air because the air inside the balloon is heated, making it less dense than the air outside the balloon.

Weight: Weight measures the amount of gravitational pull between an object and a planet, in this case, earth. Gravity is a force, or a pull, acting between two objects. The force of gravity acting on an object depends on the object's mass. The higher the mass, the higher the weight. However, the gravitational force also changes, like if you go to a different planet or the moon, so the higher the gravitational force, the higher the weight. The standard metric unit for weight is the Newton (N). Different countries use different units to measure weight, like Pounds (lb) is the US. An object with a mass of 1kg weighs 9.8 Newtons on earth and only about 1.6 Newtons on the moon.